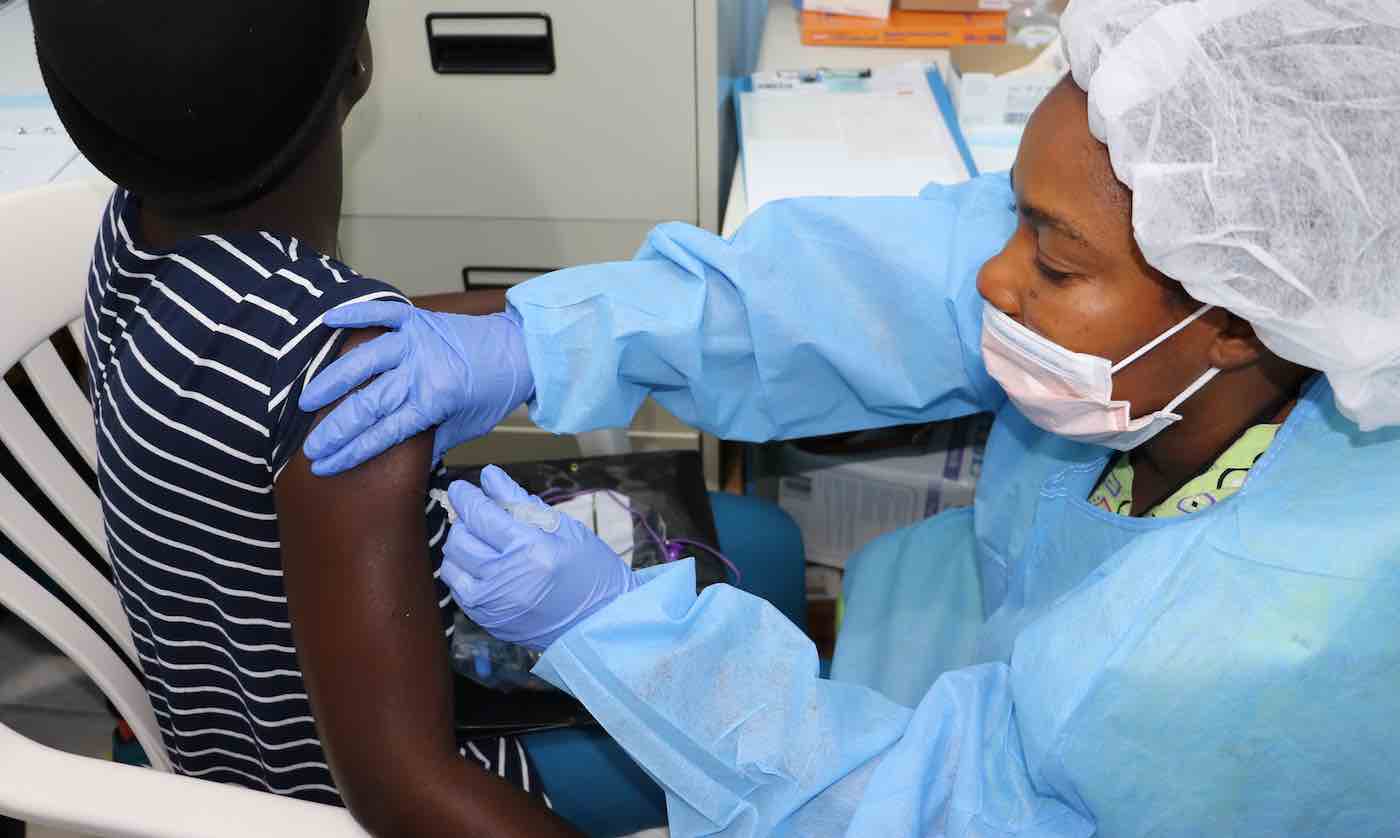
The world finally has its first ever approved Ebola vaccine.
Within 48 hours of the European Commission approving the vaccine earlier this week, the World Health Alliance (WHO) verified that the treatment also reached its health and safety standards for global use, making it the first vaccine with clinical efficacy to protect individuals 18 years of age or older at risk of infection with the Ebola virus.
The Ervebo vaccine, which was developed by pharmaceutical company Merck, has already been used to quell emergency outbreaks in the Democratic Republic of the Congo (DRC) and several other neighboring countries.
Data from clinical trials and compassionate use protocols have shown that Ervebo protects against Ebola virus disease in humans following a single-dose administration. The vaccine is being used under “compassionate use” to protect people, including children and pregnant women, at highest risk of infection as part of a ring vaccination strategy, as well as a targeted geographical vaccination when ring vaccination is not feasible. As of this week, more than 250,000 people have been vaccinated in DRC, as well as in Burundi, Uganda, South Sudan, Guinea, and Rwanda.
RELATED: Husband-Wife Duo Has Developed ‘Gene and Cell Therapy’ Cancer Vaccine Now Being Tested on Patients
The “Gavi” Vaccine Alliance—a Geneva-based health organization funding vaccine distribution in low-income countries—announced in 2015 that they would only purchase an Ebola vaccine for distribution if it was approved by a major health group.
Gavi is now welcoming the European Commission’s conditional marketing approval for Ervebo.
“This is a vaccine with huge potential,” said Dr Seth Berkley, CEO of Gavi the Vaccine Alliance. “It has already been used to protect more than 250,000 people in the DRC and could well make major Ebola outbreaks a thing of the past.
MORE: FDA Approves the First New Cystic Fibrosis Treatment in Decades
“That’s why this is such an important milestone, paving the way for a Gavi-supported global Ebola vaccine stockpile. It’s also important to credit the unprecedented global effort from African countries that helped generate the evidence as well as Merck, WHO, donor governments, partners and regulatory agencies in making this authorization happen.”
At its next meeting in December 2019, the Gavi Board is set to make a decision on a long-term Gavi Ebola vaccine program that would include the creation of a global Ebola vaccine stockpile. This stockpile, contingent on the availability of WHO’s prequalified vaccines and SAGE recommendations for their use, will enable countries to access and rapidly deploy Ebola vaccines in response to outbreaks. On top of the stockpile, the Board will also consider, if recommended, future support for preventive vaccination of high-risk groups outside of an outbreak such as healthcare workers in countries classified as being at high risk.
The current stockpile of Ebola investigational vaccine is available in part thanks to an agreement between Gavi and the vaccine’s manufacturer, Merck. In 2015, during the West Africa Ebola outbreak, Gavi made a unique offer to all manufacturers with a vaccine in Phase I clinical trials and beyond, by offering a pre-paid commitment to procure doses of licensed vaccines as and when the vaccine became licensed and available.
In return, the manufacturer was required to commit to submit an emergency use authorization application to WHO as well as an application for licensure to a stringent health authority and ensure the availability of an emergency stockpile of 300,000 investigational doses in the event of an outbreak occurring before licensure through a donation from the manufacturer to WHO. In January 2016, an Advance Purchase Commitment between Merck and Gavi was signed, creating the stockpile of investigational doses that is being used in DRC and neighbouring countries today.
In addition to its work making the investigational vaccine stockpile available, Gavi has provided $ 15.1 million to WHO to cover operational costs for the vaccination effort, funding vaccination teams, transportation, syringes and other vaccine supplies, as well as the ultra-cold fridges which keep the vaccine at the minus 60-80°C temperatures it needs to remain effective. This also includes $ 2 million provided to fund vaccinations in neighboring countries Uganda, South Sudan, Burundi and Rwanda. An additional $13.4 million funding for the vaccination effort in DRC is currently being considered.
Merck CEO Kenneth C. Frazier is now hailing the vaccine’s approval as “a historic milestone and a testament to the power of science, innovation and public-private partnership”.
“After recognizing the need and urgency for an Ebola vaccine, many came together across sectors to answer the global call for outbreak preparedness,” Frazier said in a statement. “We at Merck are honored to play a part in Ebola outbreak response efforts and we remain committed to our partners and the people we serve.
“We also look forward to continuing to work with the FDA and the African countries on their regulatory reviews over the coming months and with the World Health Organization on vaccine pre-qualification, which will help broaden access to this important vaccine for those who need it most.”
Treat Your Friends To The Positivity By Sharing The Good News To Social Media…