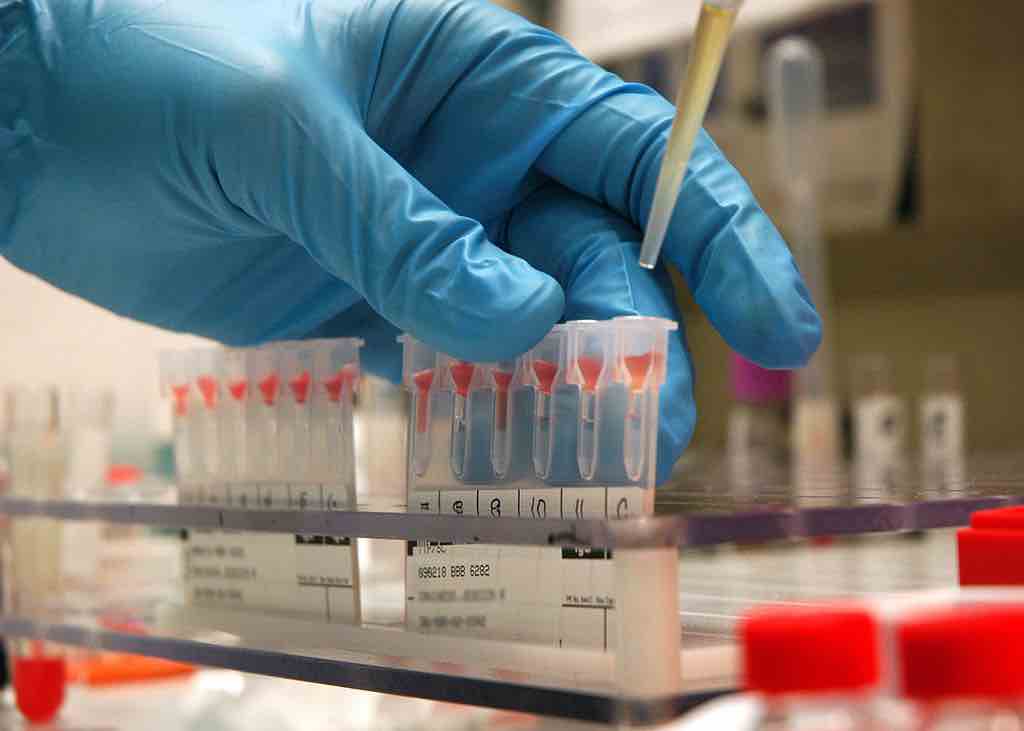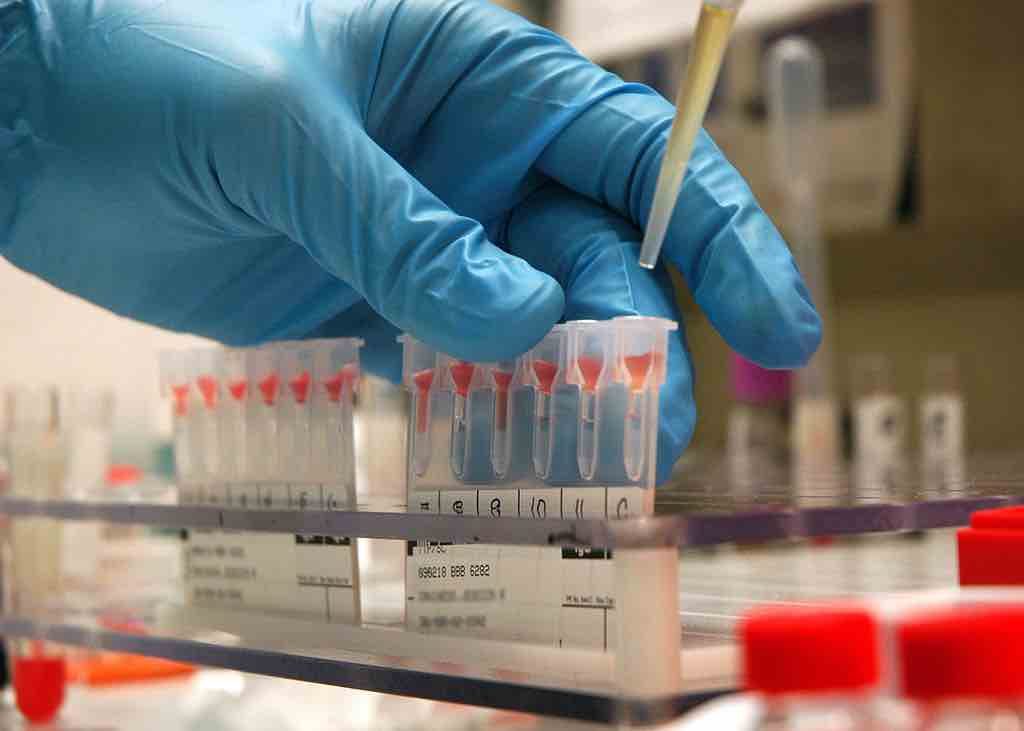
An experimental cancer test already being studied in humans shows that by examining blood proteins instead of tumor DNA, it may be possible to detect up to 18 early-stage cancers with exceptional accuracy.
Cancer tests don’t often use the same methods of detection, and having one or two unified testing options would likely save thousands of lives.
A US biotech firm called Novelna recently presented their findings of a trial of 440 humans with a total of 18 different cancers. Blood plasma samples were taken from each patient, along with 44 healthy blood donors.
By analyzing trace proteins in the blood, the Novelna team were able to achieve a high “sensitivity,” or the detection rate of early-stage tumors, and a high “specificity” or the control for false-positives. Furthermore, the proteins controlled for in the test are sex-specific.
At stage I (the earliest cancer stage) and at the specificity of 99%, the panels were able to identify 93% of cancers among males and 84% of cancers among females.
“This finding is the foundation for a multi-cancer screening test for the early detection of 18 solid tumors that cover all major human organs of origin for such cancers at the earliest stage of their development with high accuracy,” the authors wrote in the journal BMJ Oncology. “These findings pave the way for a cost-effective, highly accurate, multi-cancer screening test that can be implemented on a population-wide scale.”
The team acknowledged the small trial size and admitted that larger trials would be needed to confirm the accuracy already established, but they also highlighted that almost all of the proteins for almost all of the cancers were present in the blood samples at very low levels, indicating the importance of such tests for catching tumors before they form.
SEE ALSO: AI Can Accurately Detect 20% More Breast Cancers than Traditional Screening by Radiologists
“If the assay performance in future, well-designed sequential studies is anywhere close to what this preliminary study suggests, then it could really be a gamechanger,” Dr. Mangesh Thorat, of the Centre for Cancer Prevention at the Wolfson Institute of Preventive Medicine, told the Guardian. He was not involved in the study.
Dr. Peter Attia, the well-known MD, science communicator, and proponent of “medicine 3.0” which places strong emphasis on prevention over treatment, said recently that in order to truly bring down mortality levels of common cancers like breast, colon, and prostate cancer, early test detection should start in mid-life as often as twice a year.
MORE TESTING INNOVATIONS: Vaccine Targeting Triple-Negative Breast Cancer Shows Good Response in First Clinical Trial of Patients
Indeed, the rate of survival for women who catch breast cancer in its earliest stages is in the ninetieth percentile, while for those who catch it at stage 4, it’s very low.
Tests that would cover a variety of cancers at early stages could facilitate wider testing regimens around the world, where cancer is now responsible for one out of every six deaths.
SHARE This “Gamechanger” Cancer Test On Social Media…