(Originally published by Knowable Magazine – written by Sneha Khedkar)
Long overlooked, menstrual stem cells could be the source of important medical applications.
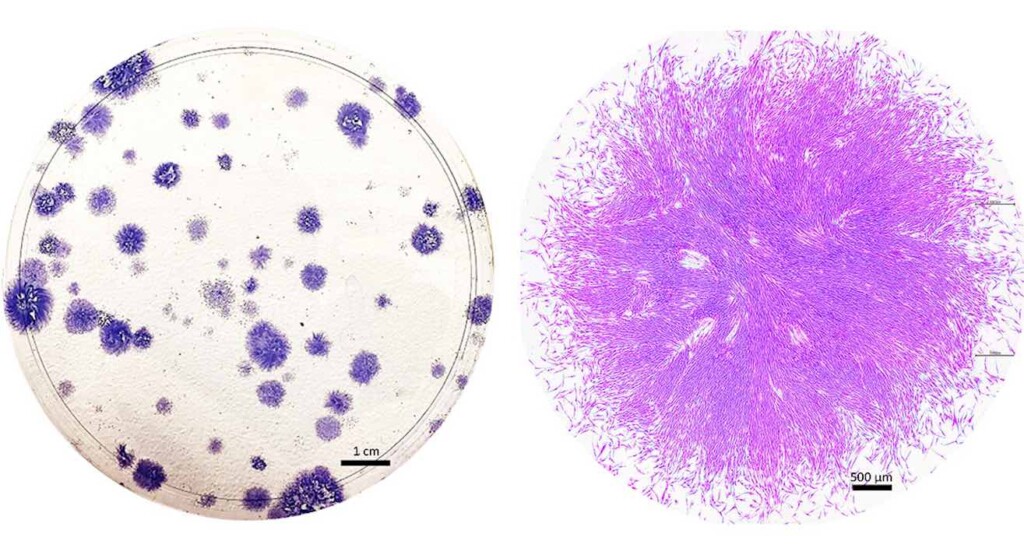
Roughly 20 years ago, a biologist named Caroline Gargett went in search of some remarkable cells in tissue that had been removed during hysterectomy surgeries. The cells came from the endometrium, which lines the inside of the uterus. When Gargett cultured the cells in a petri dish, they looked like round clumps surrounded by a clear, pink medium.
But examining them with a microscope, she saw what she was looking for — two kinds of cells, one flat and roundish, the other elongated and tapered, with whisker-like protrusions. Gargett strongly suspected that the cells were adult stem cells — rare, highly valued, self-renewing cells, some of which can become many different types of tissues.
She and other researchers had long hypothesized that the endometrium contained stem cells, given its remarkable capacity to regrow itself each month. The tissue, which provides a site for an embryo to implant during pregnancy and is shed during menstruation, undergoes roughly 400 rounds of shedding and regrowth before a woman reaches menopause.
But although scientists had isolated adult stem cells from many other regenerating tissues — including bone marrow, the heart, and muscle — “no one had identified adult stem cells in endometrium,” Gargett says.
Such cells are highly valued for their potential to repair damaged tissue and treat diseases such as cancer and heart failure. But they exist in low numbers throughout the body, and can be tricky to obtain, requiring surgical biopsy, or extracting bone marrow with a needle. The prospect of a previously untapped source of adult stem cells was thrilling on its own, says Gargett. And it also raised the exciting possibility of a new approach to long-neglected women’s health conditions such as endometriosis.
Before she could claim that the cells were truly stem cells, Gargett and her team at Monash University in Australia had to put them through a series of rigorous tests. First, they measured the cells’ ability to proliferate and self-renew, and found that some of them could divide into about 100 cells within a week. They also showed that the cells could indeed differentiate into endometrial tissue, and identified certain telltale proteins that are present in other types of stem cells.
Holistic Approach to Endometriosis: THC Holds Promise As Possible First-of-Kind Treatment for Women With Endometriosis
Gargett, who is now also with the Hudson Institute of Medical Research, and her colleagues went on to characterize several types of self-renewing cells in the endometrium. But only the whiskered cells, called endometrial stromal mesenchymal stem cells, were truly “multipotent,” with the ability to be coaxed into becoming fat cells, bone cells, or even the smooth muscle cells found in organs such as the heart.
Around the same time, two independent research teams made another surprising discovery: Some endometrial stromal mesenchymal stem cells could be found in menstrual blood.
Gargett was surprised that the body would so readily shed its precious stem cells. Since they are so important for the survival and function of organs, she didn’t think the body would “waste” them by shedding them. But she also recognized the finding’s significance: Rather than relying on an invasive surgical biopsy to obtain the elusive stem cells she’d identified in the endometrium, she could collect them via menstrual cup.

Gargett’s team has shown that these special stem cells are present in both the lower and upper layers of the endometrium. The cells are typically wrapped around blood vessels in a crescent shape, where they are thought to help stimulate vessel formation and play a vital role in repairing and regenerating the upper layer of tissue that gets shed each month during menstruation.
CHECK OUT: The Feminine Reason for African Girls Dropping Out, and An Engineer’s Simple Solution
This layer is crucial to pregnancy, providing support and nourishment for a developing embryo. The layer, and the endometrial stem cells that prod its growth, also appears to play an important role in infertility: An embryo can’t implant if the layer doesn’t thicken enough.
Endometrial stem cells have also been linked to endometriosis, a painful condition that affects roughly 190 million women and girls worldwide. Although much about the condition isn’t fully understood, researchers hypothesize that one cause is the backflow of menstrual blood into a woman’s fallopian tubes. Endometrial stem cells that get deposited in these areas may cause endometrial-like tissue to grow outside of the uterus, leading to lesions that can cause excruciating pain, scarring and, in many cases, infertility.
Researchers are still developing a reliable, noninvasive test to diagnose endometriosis, and patients wait an average of nearly seven years before receiving a diagnosis.
But studies have shown that stem cells collected from the menstrual blood of women with endometriosis have different shapes and patterns of gene expression than cells from healthy women. Several labs are working on ways to use these differences in menstrual stem cells to identify women at higher risk of the condition, which could lead to faster diagnosis and treatment.
Menstrual stem cells may also have therapeutic applications. Some researchers working on mice, for example, have found that injecting menstrual stem cells into the rodents’ blood can repair the damaged endometrium and improve fertility.
Other research in lab animals suggests that menstrual stem cells could have therapeutic potential beyond gynecological diseases. In a couple of studies, for example, injecting menstrual stem cells into diabetic mice stimulated regeneration of insulin-producing cells and improved blood sugar levels. In another, treating injuries with stem cells or their secretions helped heal wounds in mice.
A handful of small but promising clinical trials have found that menstrual stem cells can be transplanted into humans without adverse side effects.
LAW FOR WOMEN: New Illinois Law Allows Women to Get Birth Control Pills from a Pharmacist Without a Doctor Involved
Gargett’s team is also attempting to develop human therapies. She and her colleagues are using endometrial stem cells — those taken directly from endometrial tissue, rather than menstrual blood — to engineer a biodegradable mesh to treat pelvic organ prolapse, a common, painful condition that is often caused by childbirth.
Despite the convenience of collecting adult multipotent stem cells from menstrual blood, research exploring and utilizing the stem cells’ power — and their potential role in disease — still represents a tiny fraction of stem cell research, says Daniela Tonelli Manica, an anthropologist leading studies at Brazil’s State University of Campinas.
But, she and others have turned menstruation into an exciting new frontier in regenerative medicine; it’s not just a monthly inconvenience anymore.

This article originally appeared in Knowable Magazine, a nonprofit publication dedicated to making scientific knowledge accessible to all. Sign up for Knowable Magazine’s newsletter, here. The author, Sneha Khedkar, is a biologist and former research fellow turned freelance science journalist in Bengaluru, India.




















A “monthly inconvenience?” That is EXACTLY the type of thinking that has vilified the natural function of menstruation and prevented all kinds of healing therapies from being explored and made available to humans…
I am happy that the trend of acceptance and appreciation for Nature in all of it’s forms is growing – although slowly.