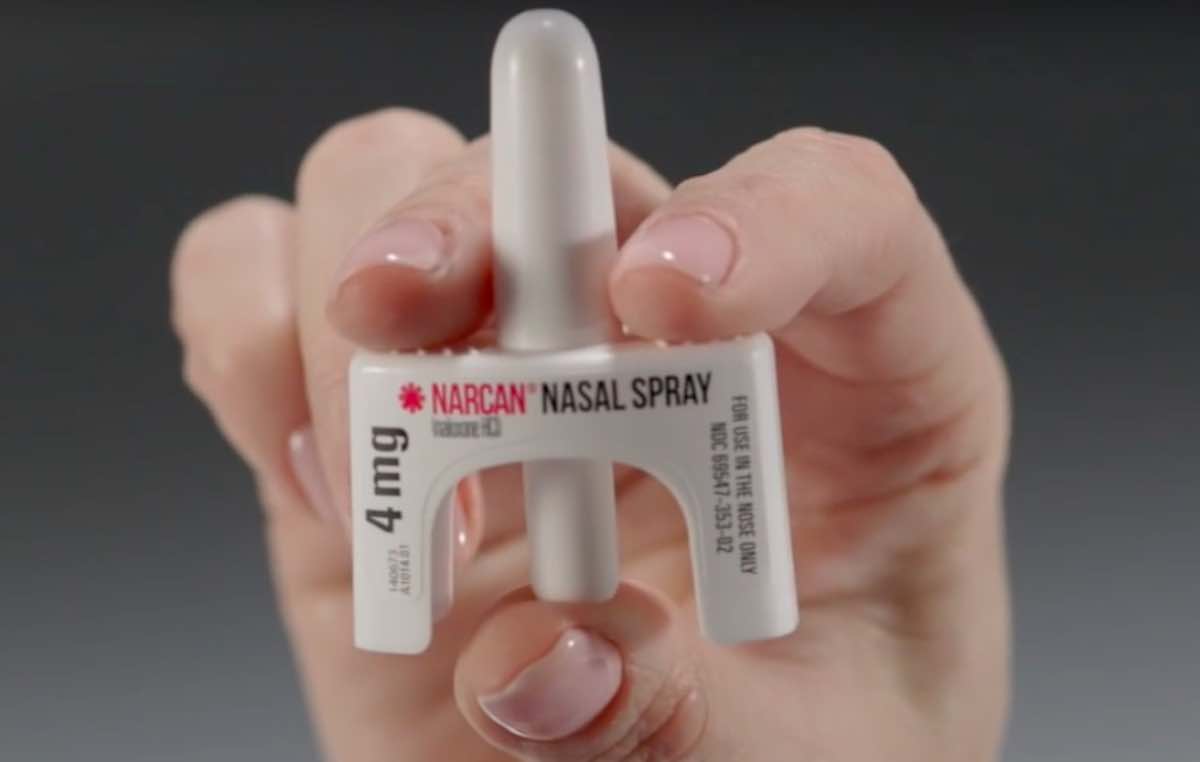
The U.S. Food and Drug Administration has approved the lifesaving drug Narcan for over-the-counter use without a prescription for the first time.
The nasal spray containing 4 milligrams of naloxone hydrochloride can rapidly reverse the effects of opioid overdose and is the standard treatment in such emergencies.
The FDA action paves the way for the life-saving doses to be sold directly to consumers in places like drug stores, convenience stores, grocery stores and gas stations, as well as online.
The timeline for availability and price will be determined by the American manufacturer, Emergent BioSolutions, but the FDA says the transition from prescription to OTC status, “may take months”.
Other formulations and dosages of naloxone will remain available by prescription only.
Drug overdose persists as a major public health issue in the United States, with more than 101,750 reported fatal overdoses occurring in the 12-month period ending in October 2022, primarily driven by synthetic opioids like illicit fentanyl.
OVERDOSE HERO: Instead of Buying New Car, Retired Paramedic Spends $40K on Overdose Prevention Kits and Already Saved 94 Lives
“The FDA has used its regulatory authority to facilitate greater access to naloxone…to address the dire public health need,” said FDA Commissioner Robert M. Califf, M.D. “Today’s approval of OTC naloxone nasal spray will increase the number of locations where it’s available and help reduce opioid overdose deaths throughout the country. We encourage the manufacturer to make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Narcan nasal spray was first approved by the FDA in 2015 as a prescription drug—and more than 44 million doses have been distributed since its public launch in 2016. In accordance with a process to change the status of a drug from prescription to nonprescription, the manufacturer provided data demonstrating that the drug is safe and effective for use as directed.
The manufacturer also showed that consumers can understand how to use the drug safely and effectively without the supervision of a healthcare professional.
In February 2023, FDA committee members voted unanimously to recommend it be approved for marketing without a prescription.
RELATED: Every US Public Library, High School, and YMCA is Getting Opioid Overdose Reversal Kits for Free
The approval of OTC Narcan nasal spray will require a change in the labeling for the currently approved 4 mg generic naloxone nasal spray products that rely on Narcan as their reference listed drug product, according to the FDA press release. Manufacturers of these generic products (which can cost around $45 for two doses—compared to Narcan at $140) will be required to submit a supplement to their applications to effectively switch their products to OTC status. The approval may also affect the status of other brand-name naloxone nasal spray products of 4 mg or less, but determinations will be made on a case-by-case basis and the FDA may contact other firms as needed.
“Naloxone is a critical tool in addressing opioid overdoses and today’s approval underscores the extensive efforts the agency has undertaken to combat the overdose crisis,” said Patrizia Cavazzoni, M.D., director of the FDA’s Center for Drug Evaluation and Research. “The FDA is working with our federal partners to help ensure continued access to all forms of naloxone during the transition of this product from prescription status to nonprescription/OTC status.
“Further, we will work with any sponsor seeking to market a nonprescription naloxone product, including through an Rx to OTC switch, and encourage manufacturers to contact the agency as early as possible to initiate discussions.”
ANNOUNCE The Remedy With Friends and Family on Social Media…