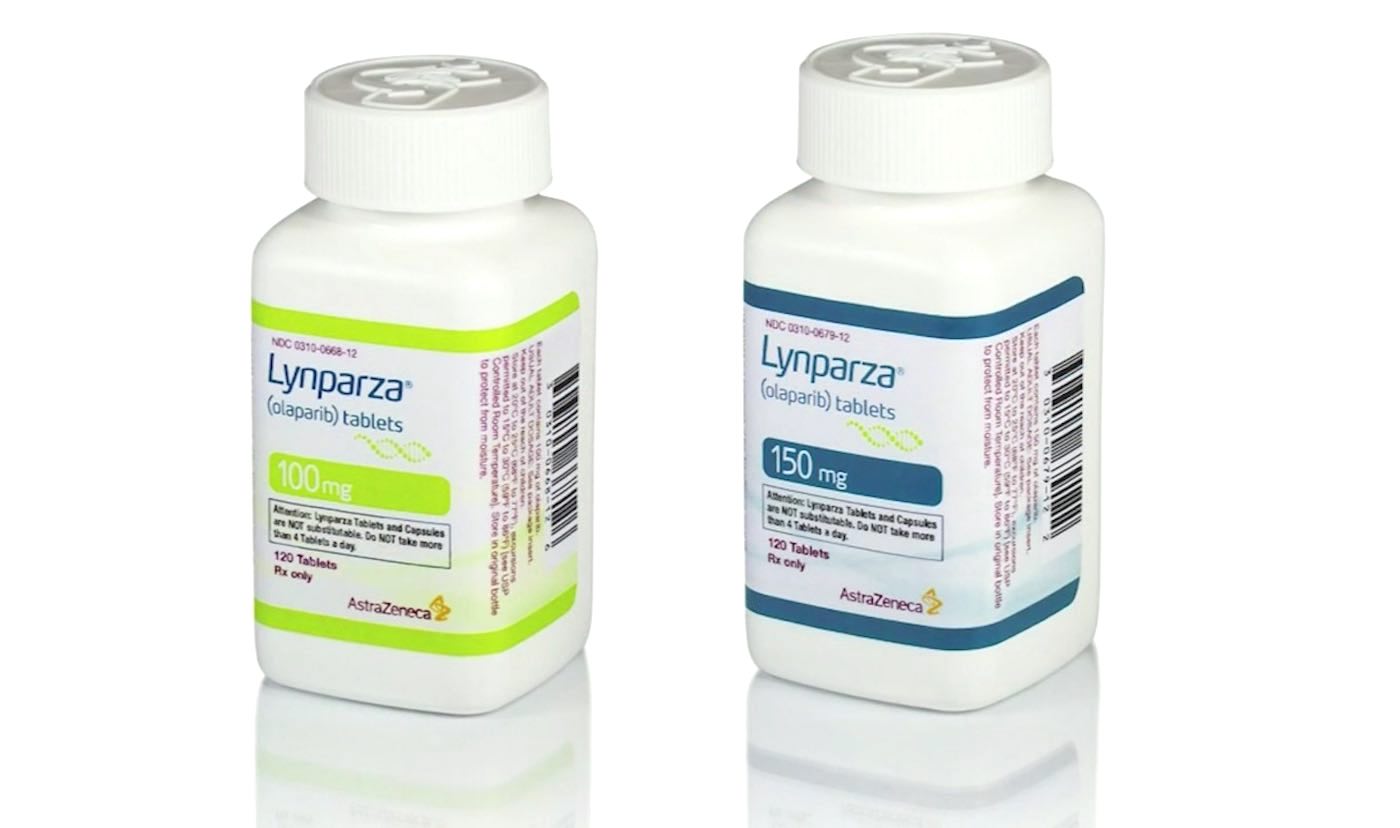
Earlier this week, the FDA approved an ovarian cancer drug as a new maintenance treatment for advanced pancreatic cancer—a notoriously dangerous form of cancer.
The drug, Lynparza (olaparib) was developed by pharmaceutical companies AstraZeneca and Merck & Co as a maintenance treatment for patients with a specific gene mutation whose cancer spread beyond the pancreas and whose disease did not progress after at least 16 weeks of chemotherapy. Patients will now be selected for therapy based on an FDA-approved companion diagnostic for Lynparza.
The approval follows the recommendation from the US FDA Oncologic Drugs Advisory Committee last month for Lynparza in this indication, and was based on results from the pivotal Phase III POLO trial published in The New England Journal of Medicine.
POLO is a Phase III randomized, double-blinded, placebo-controlled, multi-centre trial of Lynparza tablets (300mg twice daily) as maintenance monotherapy versus placebo. The trial randomized 154 patients with the aforementioned disease traits to receive either Lynparza or placebo until disease progression. The endpoints of the trial included overall survival, time to second disease progression, overall response rate and health-related quality of life.
Results showed a statistically significant and clinically meaningful improvement in progression-free survival, where Lynparza nearly doubled the time patients with gBRCAm metastatic pancreatic cancer lived without disease progression or death. The safety and tolerability profile of Lynparza in the POLO trial was in line with that observed in prior clinical trials.
“Patients with advanced pancreatic cancer historically have faced poor outcomes due to the aggressive nature of the disease and limited treatment advances over the last few decades,” said Dave Fredrickson, Executive Vice President of the Oncology Business Unit. “Lynparza is now the only approved targeted medicine in biomarker-selected patients with advanced pancreatic cancer.”
Pancreatic cancer is a deadly cancer with a high unmet medical need. It is the 12th most commonly occurring cancer and the 7th leading cause of cancer death globally. The disease has the lowest survival rate of the most common cancers and is the only major cancer with a single-digit five-year survival rate (2-9%) in nearly every country. As there are often no symptoms, or symptoms may be non-specific in the early stages, it is most commonly diagnosed at an incurable stage. Around 80% of pancreatic cancer patients are diagnosed when the disease has metastasized and for these, the average survival is less than a year.
MORE: FDA Approves the First New Cystic Fibrosis Treatment in Decades
Despite advances in treatment, few improvements have been made in diagnosis and treatment over the decades. Current treatment is surgery (for which approximately only 10-20% of patients are eligible), chemotherapy and radiotherapy, highlighting a critical unmet medical need for more effective treatment options.
Pancreatic Cancer Action Network President and CEO Julie Fleshman, said: “Metastatic pancreatic cancer patients have been waiting a long time for new therapy options for their devastating disease. Today’s approval of Lynparza provides an exciting new treatment option for patients with germline BRCA-mutated metastatic pancreatic cancer.”
Lynparza is currently approved in several dozen countries for the maintenance treatment of various forms of ovarian and breast cancer and it has been used in over 25,000 patients worldwide. Lynparza also has the broadest and most advanced clinical trial development program of any PARP inhibitor drug.
Cure Your Friends Of Negativity By Sharing The Exciting News To Social Media…