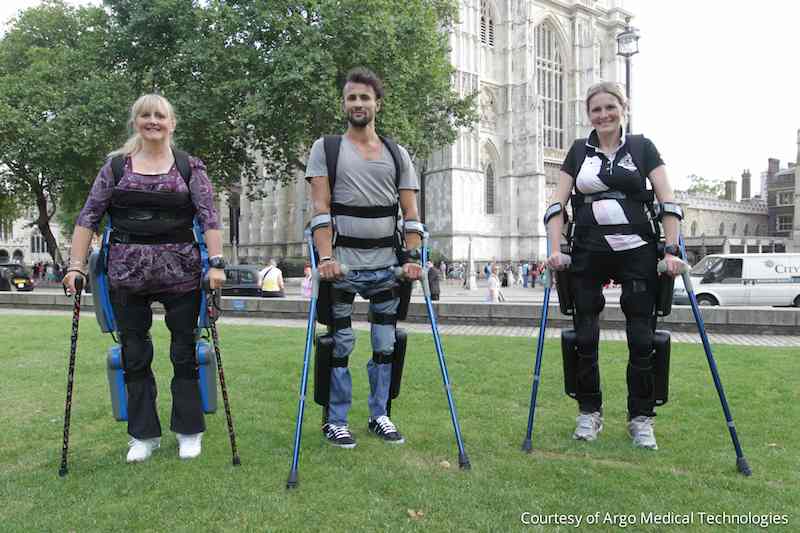
A robotic exoskeleton that allows some people with spinal cord injuries to walk upright has been approved by the U.S. Federal Drug Administration and by Health Canada earlier this summer for marketing to consumers.
Developed in Israel the ReWalk system is the first motorized device intended to act as an exoskeleton for people with lower body paralysis (paraplegia) due to a spinal cord injury. The device is worn over the legs and part of the upper body and uses a wireless remote control worn on the wrist, to command ReWalk to stand up, sit down or walk.
“Innovative devices such as ReWalk go a long way towards helping some of the more than 200,000 individuals with spinal cord injuries in the United States gain some mobility,” said Christy Foreman, director of the Office of Device Evaluation, at the FDA’s Center for Devices and Radiological Health. “Along with physical therapy, training and assistance from a caregiver, these individuals may be able to use these devices to walk again in their homes and in their communities.”
ReWalk consists of a fitted, metal brace that supports the legs and part of the upper body; motors that supply movement at the hips, knees, and ankles; a tilt sensor; and a backpack that contains the computer and power supply. Crutches provide the user with additional stability when walking, standing, and rising up from a chair.
“What I love the most,” Dan Webb told the Philadelphia Inquirer, “is looking at people eye to eye.”
ReWalk-P costs $69,500 and is not yet covered by insurance but company officials say they are in talks with insurers, and with wider usage costs could drop.
(WATCH the video below)
SHARE This Inspiring Development With the World on Social Media…